Project Context
At RealDev, we firmly believe that every challenge is an opportunity to innovate and deliver sustainable solutions. Recently, we partnered with a key player in the manufacturing sector to address significant data integrity gaps related to sterilization equipment—autoclaves—across multiple production sites. This project, which spanned 2 years, required close collaboration with system integrators, electricians, and the equipment manufacturer, to ensure a seamless and efficient execution.
Our Mission
Our mission was clear: design and implement a reliable, secure, and centralized solution to manage operational data for 27 pieces of equipment across 8 Manufacturing Units, guaranteeing compliance, efficiency, and robust data management.
With this dedication to establish compliance with pharmaceutical standards, we carried out thorough Data Integrity (DI) assessments following ALCOA+ principles. This methodology ensures that all data is attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available.
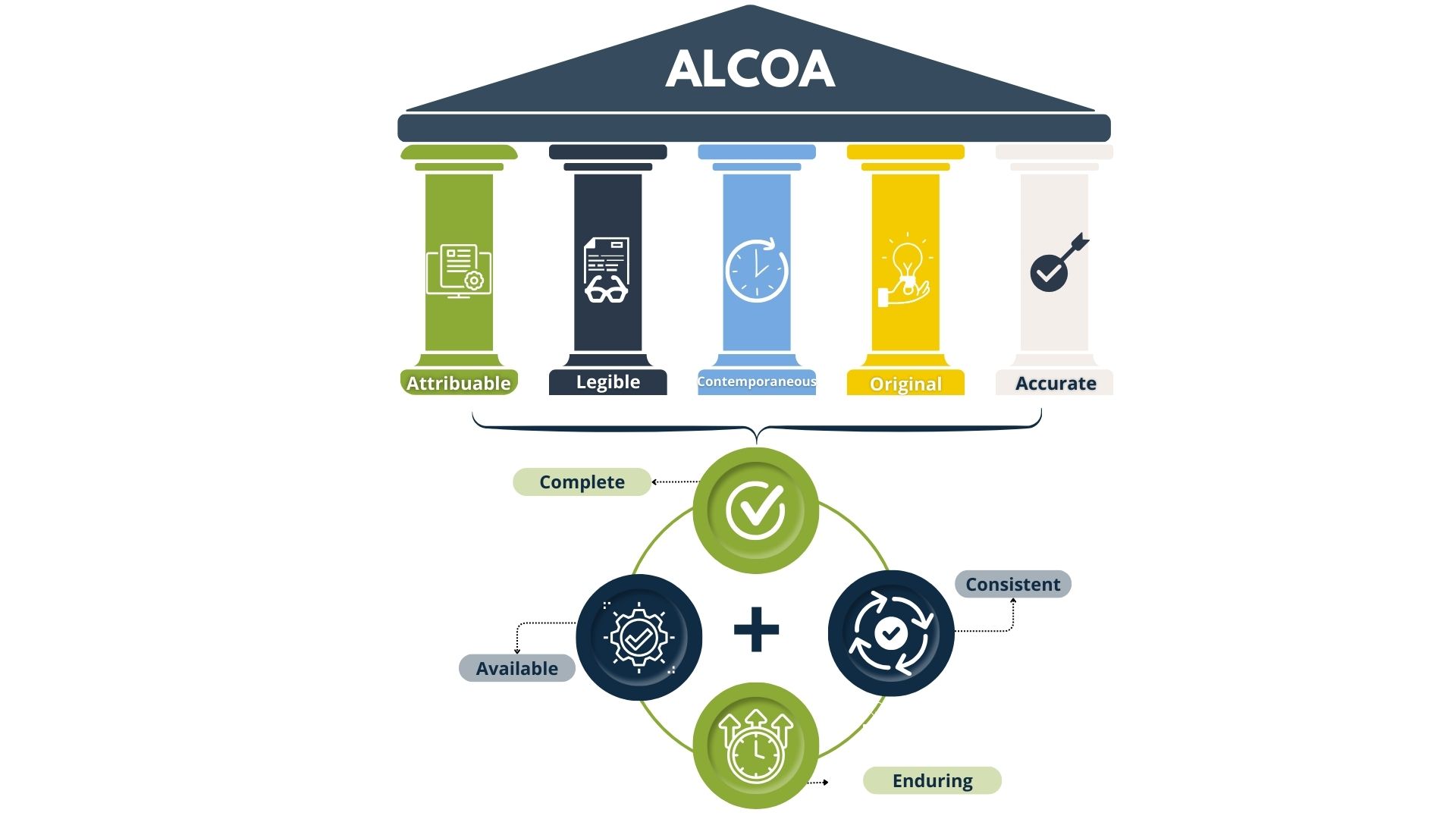
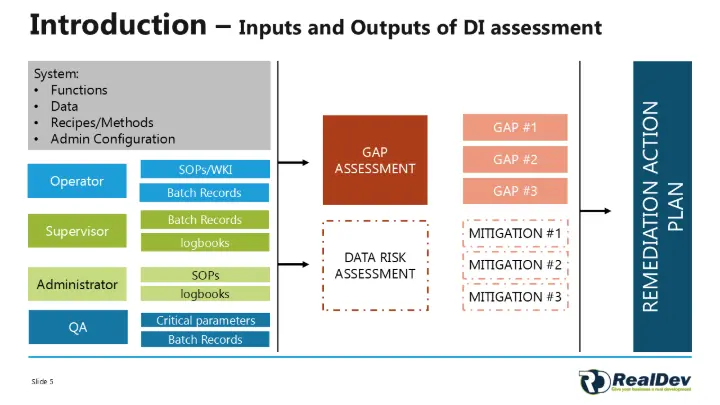
Through detailed GAP assessments and Data Risk Assessments (as illustrated in the attached diagrams), we have identified and addressed all eDI gaps. By implementing targeted remediation actions, we have aligned our processes with ALCOA+ standards, guaranteeing robust data integrity and compliance with industry regulations.
RealDev's Approach
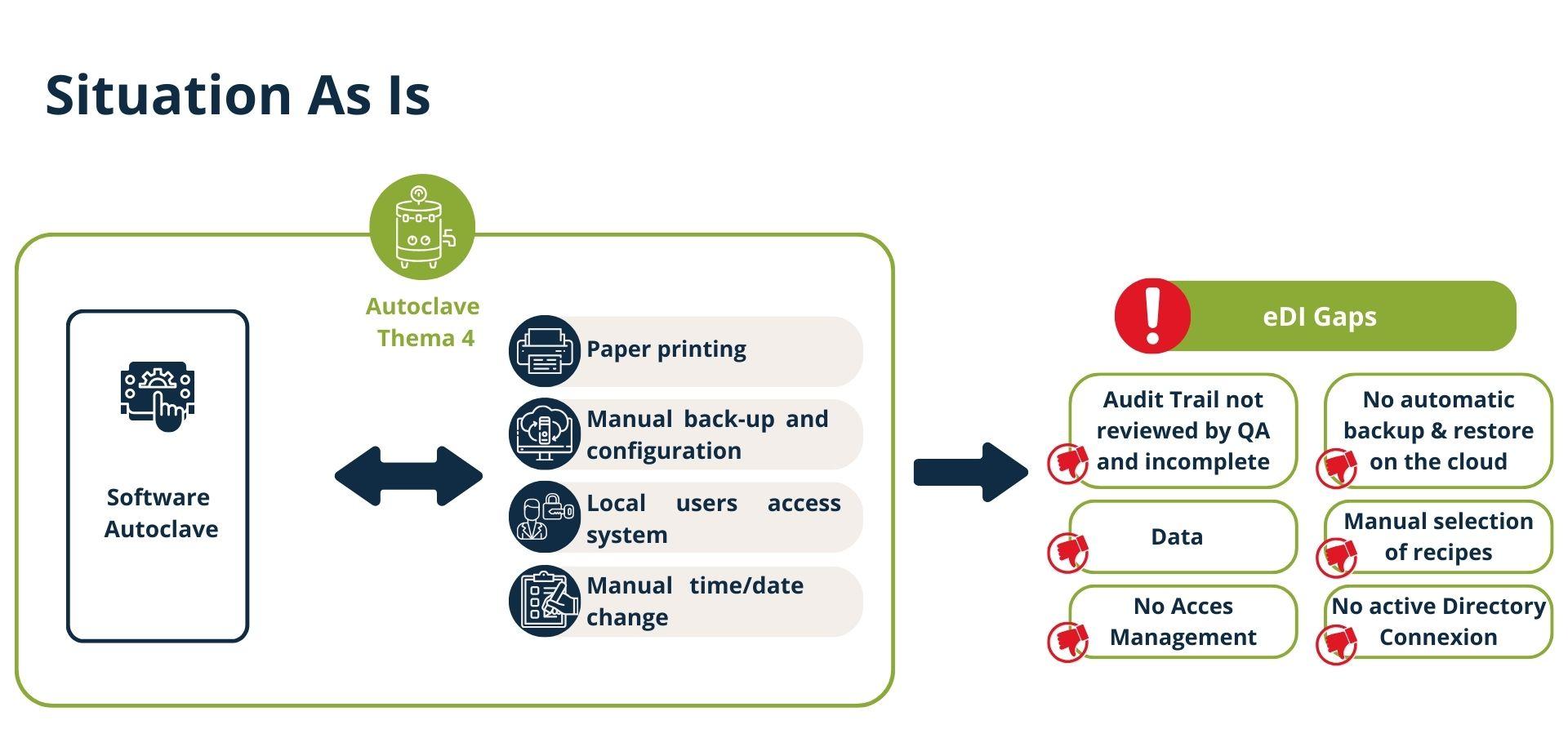
Phase 1 - Technical Study
A comprehensive assessment of the existing systems revealed several critical challenges threatening operational performance and regulatory compliance. These challenges included:
- Inadequate data backup and recovery procedures, increasing the risk of production downtime.
- Absence of audit trails compliant industry standards, compromising traceability and conformity.
- Insufficient access controls and data validation processes.
- Lack of synchronized time servers (NTP) across equipment, creating inconsistencies in production logs.
These issues not only impacted data reliability but also posed risks to product quality, operational continuity, and organizational reputation.
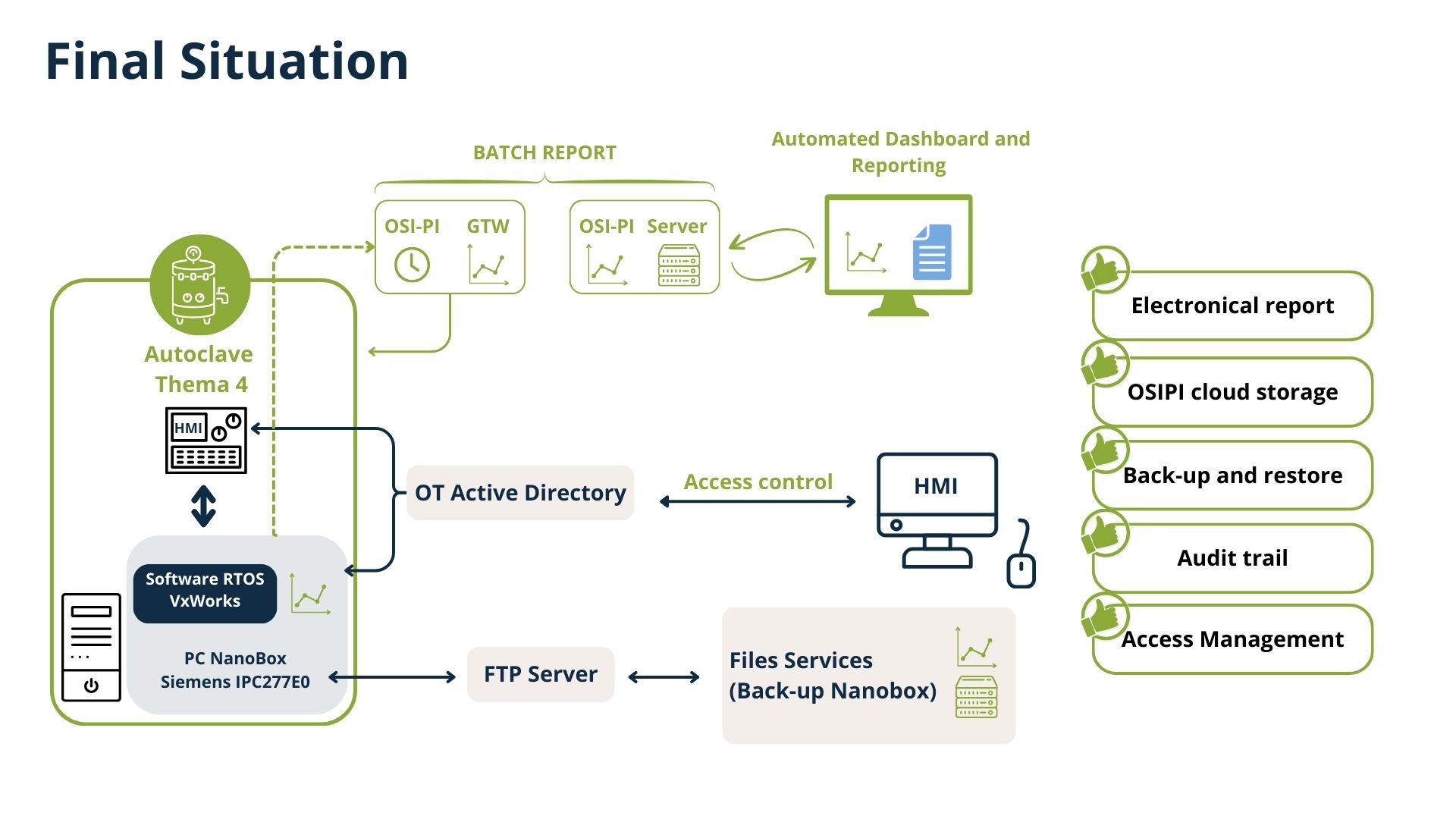
Phase 2 – Commissioning & full-Scale Implementation & IQOQ validation
Building on lessons learned from the pilot phase, RealDev rolled out the solution across the 27 autoclaves spanning 8 Manufacturing Units. The success of this phase was made possible through close collaboration with integrators, electricians, and the equipment manufacturer, aligning technical execution with operational realities.
Key activities included:
Stakeholder Coordination
Ensuring fluid communication between integrators, clients, and partners including the installation of electrical wiring, network data cables from IT (information technology) to OT (operational technology - Cyber security), and the new power supply for the screen.
Replacement
Replacement of the existing HMI screen with a Phoenix Contact Screen
Technical Execution
Deploying hardware (Nanobox) and software for automatic data transfer towards OsiPI (historical database) and enabling real-time monitoring.
Centralized Data Collection
A robust system capable of securely capturing and storing critical cycle parameters — time, temperature, and pressure — in real time.
Synchronization
Implementing NTP servers to ensure time consistency across all equipment.
Automated Backup and Audit Trail:
Ensuring that all data is reliably stored, traceable, and compliant with industry standards (e.g., FDA, GMP).
Qualification & Compliance
Validating all systems to meet industry standards for data integrity and operational performance.
Training
Providing comprehensive training for production and maintenance teams to ensure adoption and smooth operations.

Phase 3 - Project Closure
The final phase focused on knowledge transfer and continuous improvement:
- User Training: Development of detailed user guides and hands-on training for operators.
- Review and Feedback: Post-action review meetings to ensure alignment with project goals and gather stakeholder insights.
- Ongoing Support: RealDev remains committed to supporting the client in optimizing system performance and addressing any future needs.
Key Benefits
By addressing data integrity and operational inefficiencies, RealDev delivered tangible improvements, including:
- Enhanced Compliance: Reliable audit trails and synchronized data ensure compliance with industry regulations.
- Operational Efficiency: Centralized data collection, backup, and real-time monitoring optimize production processes.
- Data Integrity: Ensuring accurate, secure, and validated data across 27 autoclaves for consistent product quality.
- Collaborative Success: A project delivered in close partnership with integrators, electricians, and the equipment manufacturer over a 2-year period.
- Future-Readiness: Scalable architecture supporting growth and additional equipment integration.
Conclusion
This project reflects RealDev’s commitment to delivering cutting-edge solutions tailored to client needs. By addressing critical gaps, we ensured robust data integrity, optimized operations, and improved decision-making capabilities.
At RealDev, we don’t just solve problems, we transform them into opportunities for growth and innovation. Partner with us today to unlock the full potential of your operations while ensuring compliance, reliability, and performance across your equipment.






